


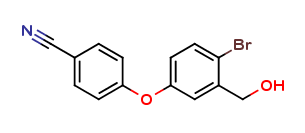
We are 4-(4-bromo-3-(hydroxyMethyl)phenoxy)benzonitrile CAS:906673-45-8 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Because API companies usually adopt a different synthetic route from the original research company, compared with the drug itself, the impurities contained in the generic drug API have not been verified by the original drug for many years of use and require more careful testing and control.5,6,7,8-Tetrahydroquinoxaline CAS:34413-35-9 Trial results demonstrated that Oligomannate statistically improved cognitive function in mild-to-moderate AD patients as early as week 4 and the benefit was sustained at each follow-up assessment visit,” Shanghai Green Valley Pharmaceuticals, which developed the drug along with two academic institutions in China, said in a statement.1-bromo-5-yodopentano CAS:88962-86-1 Trial results demonstrated that Oligomannate statistically improved cognitive function in mild-to-moderate AD patients as early as week 4 and the benefit was sustained at each follow-up assessment visit,” Shanghai Green Valley Pharmaceuticals, which developed the drug along with two academic institutions in China, said in a statement.Ácido 2-amino-4-fluorobenzoico CAS:446-32-2 API (Active Pharmaceutical Ingredient) means the active ingredient which is contained in medicine.The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.
| Product Name | |
|---|---|
| 1,3,5-benzenetricarboxylic acid chloride Cas:4422-95-1 | View Details |
| Germanium Tetraiodide Cas:13450-95-8 | View Details |
| 2-Fluoro-1,3-dimethylimidazolidinium hexafluorophosphate | View Details |