


We are Upadacitinib CAS:1310726-60-3 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description
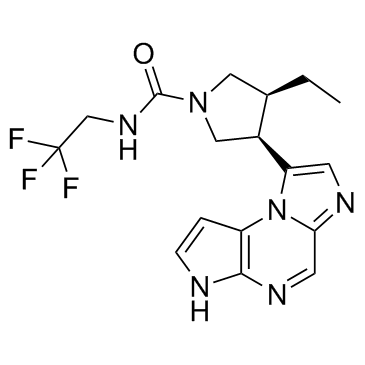
Product Name: Upadacitinib CAS 1310726-60-3
Synonyms:
Upadacitinib;
UNII-4RA0KN46E0;
(3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-1-pyrrolidinecarboxamide;
(3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide;
ABT-494; ABT 494;
(3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-1-pyrrolidinecarboxamide;
1-Pyrrolidinecarboxamide, 3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-, (3S,4R)-;
rel-(-)-(3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide;
Chemical & Physical Properties
Appearance : White to off-white powder.
Assay : ≥99.0%
Density:1.6±0.1 g/cm3
Upadacitinib is an oral Janus kinase (JAK)1-selective inhibitor and a disease-modifying antirheumatic drug (DMARD) used in the treatment of rheumatoid arthritis to slow down disease progression. Rheumatoid arthritis is a chronic autoimmune inflammatory disease affecting the peripheral joints. It is characterized by synovial inflammation and hyperplasia, autoantibody production, cartilage damage and bone destruction, leading to co-morbidities. Despite a variety of therapeutic agents available for treatment, up to 40% of the patients do not respond to current therapies, including biological therapies. The etiology of the disease is mostly unknown; however, the role of JAK as a driver of immune-mediated conditions was discovered, leading to the use of JAK as therapeutic targets for rheumatoid arthritis. To reduce dose-related toxicity (as seen with some pan-JAK inhibitors) without significantly affecting efficacy, more selective JAK1 inhibitors, upadacitinib and [filgotinib], were developed. The FDA approved Upadacitinib in August 2019 for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate. In December 2019, it was additionally approved by the European Commission for the same indication in patients with inadequate response or intolerance to one or more DMARDs and can be used as monotherapy or in combination with methotrexate. Upadacitinib is marketed under the brand name RINVOQ™ for oral administration. It is currently being investigated in several clinical trials assessing its therapeutic effectiveness in other inflammatory diseases, such as psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and atopic dermatitis.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:2-methyl-1-morpholino-propane-2-thiol manufacturer | isolongifololaldehyde-semicarbazone supplier | 4-acetyloxy-3-(3-methylbut-2-enyl)benzoic acid producer.
| Product Name | |
|---|---|
| MAGNESIUM BORIDE Cas:15996-76-6 | View Details |
| Zinc bromide liquid Cas:7699-45-8 | View Details |
| 2-Fluoro-5-bromo-iodobenzene Cas:116272-41-4 | View Details |