We are Tianeptine CAS:66981-73-5 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
CAS No: 66981-73-5
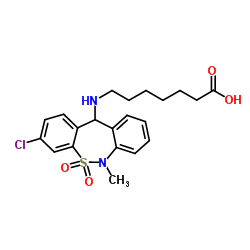
Product Name: Tianeptine
Other Name:
Tianeptine
Tianeptina
Tianeptinum
Tianeotine
7-[(3-Chloro-6-methyl-5,5-dioxido-6,11-dihydrodibenzo[c,f][1,2]thiazepin-11-yl)amino]heptanoic acid
Density: 1.4±0.1 g/cm3
Boiling Point: 609.2±65.0 °C at 760 mmHg
Melting Point: 129-131°C
Molecular Formula: C21H25ClN2O4S
Molecular Weight: 436.952
Flash Point: 322.2±34.3 °C
Exact Mass: 436.122345
PSA: 95.09000
LogP: 2.57
Vapour Pressure: 0.0±1.8 mmHg at 25°C
Index of Refraction: 1.639
Specification
Appearance: White powder
Assay: ≥99.0%
Residue on ignation: ≤0.20%
Loss on drying: ≤1.0%
Heavy metal: ≤20ppm
Individual impurity: ≤0.1%
Total impurities: ≤0.5%
Application
Mainly acting on the 5-HT system, no excitement, sedation, anti-acetylcholine and cardiac toxicity. For depression.
Tianeptine (brand names Stablon, Coaxil, Tatinol, Tianeurax and Salymbra) is a drug used primarily in the treatment of major depressive disorder, although it may also be used to treat asthma or irritable bowel syndrome. Chemically it is a tricyclic antidepressant (TCA), but it has different pharmacological properties than typical TCAs as recent research suggests that tianeptine produces its antidepressant effects through indirect alteration of glutamate receptor activity (i.e., AMPA receptors and NMDA receptors) and release of BDNF, in turn affecting neural plasticity.
Tianeptine has antidepressant and anxiolytic (anti-anxiety) properties with a relative lack of sedative, anticholinergic and cardiovascular adverse effects, thus suggesting it is particularly suitable for use in the elderly and in those following alcohol withdrawal; such persons can be more sensitive to the adverse effects of psychotropic drugs. Recent results indicate possible anticonvulsant (anti-seizure) and analgesic (painkilling) activity of tianeptine via immediate or downstream modulation of adenosine A1 receptors (as the effects could be experimentally blocked by antagonists of this receptor).
Tianeptine is a low-affinity full agonist at the μ-opioid[note 1] and δ-opioid receptors with negligible effect at the κ-opioid receptors. μ-Opioid agonists typically induce euphoria, and in accordance, tianeptine does so at high doses well above the normal therapeutic range.
Tianeptine was discovered and patented by The French Society of Medical Research in the 1960s. Currently, tianeptine is approved in France and manufactured and marketed by Laboratories Servier SA; it is also marketed in a number of other European countries under the trade name “Coaxil” as well as in Asia (including Singapore) and Latin America as “Stablon” and “Tatinol” but it is not available in Australia, Canada, New Zealand, the U.K. or the U.S..
Package: 25kg/drum, can also be designed according to customer requirements.
Storage: Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Related News: “Our collaboration with Mankind demonstrates our commitment towards providing novel, innovative treatment options for efficient diabetes management,” Glenmark Pharmaceuticals President, India Formulations, Middle East and Africa Sujesh Vasudevan said.2,6-Difluorobenzoyl isocyanate CAS:60731-73-9 Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.3-oxo-5-sulfanyl-1,2-thiazole-4-carboxylic acid,sodium Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.2-amino-3-hidroxipiridina CAS:16867-03-1 Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.Rigosertib is a small molecule that inhibits cellular signaling in cancer cells by acting as a RAS mimetic. Current clinical development of rigosertib is centered upon the therapeutic management of MDS, a heterogeneous group of bone marrow disorders characterized by ineffective hematopoiesis that often develop into acute myeloid leukaemia (AML).



