We are Remdesivir CAS:1809249-37-3 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
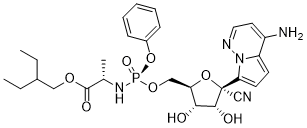
Product Name: Remdesivir
Synonym: Remdesivir; GS-5734; GS 5734; GS5734, Prodrug of GS-441524; Prodrug of GS441524; Prodrug of GS441524;
CAS#: 1809249-37-3
Chemical Formula: C28H36N5O8P
Exact Mass: 601.2301
Molecular Weight: 601.5968
IUPAC/Chemical Name: 2-ethylbutyl ((S)-(((2R,3S,4R,5R)-5-(4-aminopyrrolo[1,2-b]pyridazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)-L-alaninate
Application:
Remdesivir has been found to show reasonable antiviral activity against more distantly related viruses such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-coronavirus and Coronavirus. GS-5734 was rapidly pushed through clinical trials due to the 2013–2016 West African Ebola virus epidemic crisis.
Gilead Sciences has recently confirmed that it is demonstrating whether the experimental Ebola drug Remdesivir may cause a fight against a new coronavirus (2019-nCoV) emerging in China. The company said that "active discussions are underway with researchers and clinicians in the United States and China about the potential use of Remdesivir in therapy."
Remdesivir, also known as GS-5734, is an experimental RNA polymerase inhibitor developed by Gilead Sciences in 2014 in collaboration with the Centers for Disease Control and Prevention (CDC) and the U.S. Army Medical Institute for Infectious Diseases Anthony Fossie, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH), stated in a comment on JAMA Network that SARS-CoV and MERS-CoV are being used as prototypes to develop treatment strategies for 2019-nCoV. "Broad-spectrum antivirals, such as Remdesivir, lopinavir / ritonavir and interferon, have shown promise against MERS-CoV in animal models and are evaluating their activity against 2019-nCoV," he said.
Related News: Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.3-dimethoxyphosphoryl-3H-2-benzofuran-1-one CAS:61260-15-9 Commonly used generic drugs: the products whose patents have expired and the generic drugs of many companies have participated in the competition, and the market demand is large.6630-33-7 Commonly used generic drugs: the products whose patents have expired and the generic drugs of many companies have participated in the competition, and the market demand is large.630125-49-4 Commonly used generic drugs: the products whose patents have expired and the generic drugs of many companies have participated in the competition, and the market demand is large.Commonly used generic drugs: the products whose patents have expired and the generic drugs of many companies have participated in the competition, and the market demand is large.



