


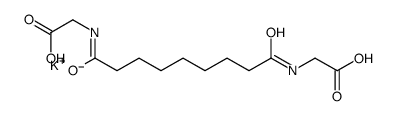
We are Potassium azeloyl diglycinate CAS:477773-67-4 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Huahai started with the development of characteristic APIs and pharmaceutical intermediates. At the same time, using the international cooperation platform and the opportunity of the expiry of patent protection of major international original research drugs, Huahai has extended the industrial chain to downstream high value-added preparations, and formed intermediates and materials The complete industrial chain of medicine and preparation integration.Cloruro de 3-cloropivaloilo CAS:4300-97-4 The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.3388-04-3 There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space.1,2,2,3-Tetrachloropropane In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.A GSK spokesman said it was not yet clear by when ViiV would be able to address the FDA’s concerns.
| Product Name | |
|---|---|
| dl-sulfOraphane Cas:4478-93-7 | View Details |
| metronidazole Cas:443-48-1 | View Details |
| Cupric Chloride Anhydrous | View Details |