


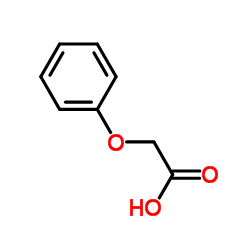
We are phenoxyacetic acid CAS:122-59-8 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Taking Teva as an example, it has 2 large R & D centers and 3 professional R & D centers in 5 countries, which effectively supports the development of Teva’s generic pharmaceutical sector.3-(9-phenyl-carbazol-3-yl)-9H-carbazole CAS:1060735-14-9 This is called API. A small amount of the active ingredient has an effect, so only a tiny part of the active ingredient is contained in medicine. N- [5-bromometil-4- (4-fluorofenil) -6-isopropilpirimidina-2-il] -N-metilmetano sulfonamida CAS:799842-07-2 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.2-Isopropylthioxanthone This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.
| Product Name | |
|---|---|
| 1-Ethylimidazole | View Details |
| 2-Ethyl-3-methylpyrazine Cas:15707-23-0 | View Details |
| n-ethyl-n-cyanoethyl-m-toluidine Cas:148-69-6 | View Details |