


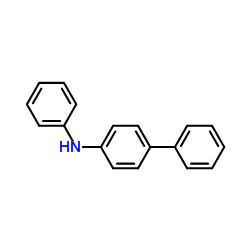
We are N,4-diphenylaniline CAS:32228-99-2 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: According to statistics, in 2017, China’s total production of chemical drugs reached 3.478 million tons, a year-on-year increase of 1.6%. The main business income showed a steady increase, from 328.972 billion yuan in 2012 to 573.475 billion yuan in 2017. The total profit was 48.644 billion yuan, but profit margins remain low (8.48% in 2017).2'-O-methyluridine CAS:2140-76-3 At present, the additional 300 tons of sunniamine, 300 tons of fluoxamic acid, and 200 tons of cyprodinil have been successfully put into production, and the profitability is good.13096-62-3 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.Ácido 4-etilfenilborónico CAS:63139-21-9 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.
| Product Name | |
|---|---|
| 1-Ethyl-7-nitro-1,2,3,4-tetrahydroquinoline | View Details |
| 1-bromo-9H-carbazole Cas:16807-11-7 | View Details |
| Tin(IV) Iodide Cas:7790-47-8 | View Details |