


We are N-(2-chloropyrimidin-4-yl)-2,3-dimethylindazol-6-amine CAS:444731-74-2 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
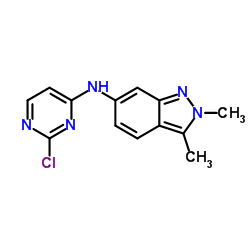
Chemical Name: N-(2-chloropyrimidin-4-yl)-2,3-dimethylindazol-6-amine
CAS.NO: 444731-74-2
Synonyms:
N-(2-chloro-4-pyrimidinyl)-2,3-dimethyl-2H-indazol-6-amine
N-(2-CHLOROPYRIMIDIN-4-YL)-2,3-DIMETHYL-2H-INDAZOL-6-AMINE
2H-Indazol-6-amine,N-(2-chloro-4-pyrimidinyl)-2,3-dimethyl
Molecular Formula: C13H12ClN5
Molecular Weight: 273.72100
Physical and Chemical Properties:
Density: 1.414g/cm3
Boiling point:549.991ºC at 760 mmHg
Melting point: /
Flash point: 286.424ºC
Refractive index: 1.705
Specification:
Appearance:White rystalline powder
Purity:≥99%
Total Impurity:1.0% Max.
LOD:1.0% Max.
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:Stored in a cool and dry well-closed container. Keep away from moisture and strong light/heat.
Application:Intermediates of Pazopanib hydrochloride CAS:635702-64-6
Related News: For example, an active ingredient to relieve pain is included in a painkiller.4-Bromo-2-trifluoromethoxyaniline For example, an active ingredient to relieve pain is included in a painkiller.2,4-Diamino phenetole sulfate CAS:68015-98-5 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.22439-61-8 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.
| Product Name | |
|---|---|
| Laurocapram | View Details |
| Bronopol Cas:52-51-7 | View Details |
| polyphosphoric acid Cas:8017-16-1 | View Details |