

We are Lansoprazole CAS:103577-45-3 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description:
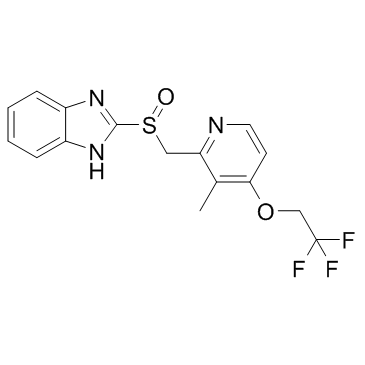
Product Name: Lansoprazole CAS NO: 103577-45-3
Synonyms:
Ketian;
Zoton;
2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methyl]sulfinyl]benzimidazole;
Chemical & Physical Properties:
Appearance: White crystalline powder
Assay :≥99.00%
Density: 1.5 g/cm3
Boiling Point: 555.8℃ at 760 mmHg
Melting Point: 178-182℃
Flash Point: 289.9℃
Refractive Index: 1.591
Storage Condition: 2-8℃
Vapor Pressure: 2.55E-10mmHg at 25℃
Solubility: Practically insoluble in water, soluble in anhydrous ethanol, very slightly soluble in acetonitrile.
Safety Information:
RTECS: DD9487500
Safety Statements: S26-S36
HS Code: 2933990090
WGK Germany: 2
Risk Statements: R36/37/38
Hazard Code: Xi
Lansoprazole is a proton-pump inhibitor (PPI) which inhibits the stomach”s production of gastric acids. It is manufactured by a number of companies worldwide under several brand names. In the United States, it was first approved by the Food and Drug Administration (FDA) in 1995. Prevacid patent protection expired on November 10, 2009. There is not evidence that its effectiveness is different than that of other PPIs.
Lansoprazole is a proton-pump inhibitor (PPI) in the same pharmacologic class as omeprazole. Lansoprazole has been marketed for many years and is one of several PPIs available. It is a racemic 1:1 mixture of the enantiomers dexlansoprazole (Dexilant, formerly named Kapidex) and levolansoprazole. Dexlansoprazole is an enantiomerically pure active ingredient of a commercial drug as a result of the enantiomeric shift.
Lansoprazole”s plasma elimination half-life (1.5 h) is not proportional to the duration of the drug”s effects to the person (i.e. gastric acid suppression). and the effects of the drug last for over 24 hours after it has been used for a day or more. Lansoprazole, given nasogastrically, effectively controls intragastric pH and is an alternative to intravenous pantoprazole in people who are unable to swallow solid-dose formulations.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:3-(6-cyclohexylhexyl)-1,3-thiazolidine,hydrochloride manufacturer | 5-(4-nitrophenyl)-1,3,8,8-tetramethyl-7,8,9,10-tetrahydropyrimido[4,5-b]quinoline-2,4,6(1H,3H,5H)-trione supplier | tert-butyl (6-(((5-ethyl-1,3,4-thiadiazol-2-yl)thio)methyl)-4-morpholinopyridin-2-yl)(3-methyl-5-nitrobenzyl)carbamate producer.
| Product Name | |
|---|---|
| 1-Chlorocarbonyl-3-Methanesulfonyl-2-Imidazolidinone | View Details |
| (9-phenyl-9H-carbazol-2-yl)boronic Acid Cas:1001911-63-2 | View Details |
| 1-ANILINONAPHTHALENE-8-SULFONIC ACID AMMONIUM SALT Cas:28836-03-5 | View Details |