


We are HYDROCHLOROTHIAZIDE CAS:58-93-5 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description:
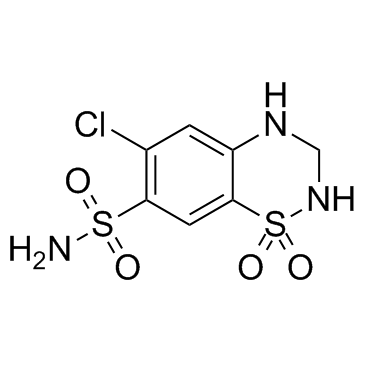
Product Name: HYDROCHLOROTHIAZIDE CAS NO: 58-93-5
Synonyms:
6-Chloro-3,4-Dihydro-(2H)-1,2,4-Benzothiadiazine-7-Sulfonamide 1,1-Dioxide;
2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide;
Chlorsulfonamidodihydrobenzothiadiazine dioxide;
Chemical & Physical Properties:
Appearance: White crystalline powder
Assay :≥99.0%
Density: 1.693g/cm3
Boiling Point: 577℃ at 760mmHg
Melting Point: 273-275℃
Flash Point: 302.7℃
Refractive Index: 1.632
Water Solubility: 722mg/L(25℃)
Stability: Stable. Incompatible with strong oxidizing agents.
Storage Condition: 2-8℃
Safety Information:
RTECS: DK9100000
Safety Statements: S22-S24/25
HS Code: 2935009090
RIDADR: UN 1230 3/PG 2
Risk Statement: R22
Hazard Codes: Xi; T; F
Hydrochlorothiazide (abbreviated HCTZ, HCT, or HZT), is a diuretic medication often used to treat high blood pressure and swelling due to fluid build up. Other uses include diabetes insipidus, renal tubular acidosis, and to decrease the risk of kidney stones in those with high calcium level in the urine. For high blood pressure it is often recommended as a first line treatment. HCTZ is taken by mouth and may be combined with other blood pressure medications as a single pill to increase the effectiveness.
Potential side effects include poor kidney function, electrolyte imbalances especially low blood potassium and less commonly low blood sodium, gout, high blood sugar, and feeling faint initially upon standing up. While allergies to HCTZ are reported to occur more often in those with allergies to sulfa drugs this association is not well supported. It may be used during pregnancy but is not a first line medication in this group.
It is in the thiazide medication class and acts by decreasing the kidneys” ability to retain water. This initially reduces blood volume, decreasing blood return to the heart and thus cardiac output. Long term, however, it is believed to lower peripheral vascular resistance.
Two companies, Merck and Ciba, state they discovered the medication which became commercially available in 1959. It is on the World Health Organization”s List of Essential Medicines, the most important medications needed in a basic health system. In 2008 it was the second most commonly used blood pressure medication in the United States. It is available as a generic drug and is relatively affordable. Labelled Hydrochlorothiazide (H714560). Hydrochlorothiazide is a carbonic anhydrase inhibitor as a diuretic.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:{NiBr2(tris-{3-dimethylarsino-propyl}-arsine)}ClO4 manufacturer | 2H-1-Benzopyran-2-one, 3-(2-chloro-4-methoxybenzoyl)- supplier | 5-O-desosaminyl-9-deoxo-9-dihydro-9a-N-[N’-(4-fluorobenzenesulfonyl) carbamoyl-γ-aminopropyl]-9a-aza-9a-homoerithronolide A producer.
| Product Name | |
|---|---|
| N-(4-Methylphenyl)-3-oxobutanamide | View Details |
| Rhenium (IV) Sulfide Cas:12038-63-0 | View Details |
| VINYLENE CARBONATE Cas:872-36-6 | View Details |