

We are DOPAC CAS:102-32-9 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description:
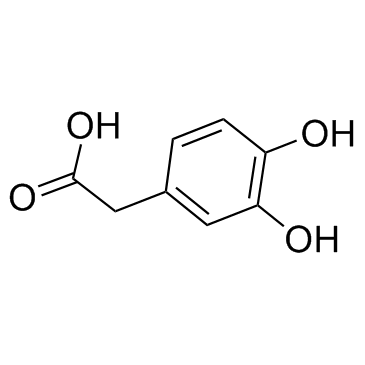
Product Name: DOPAC CAS NO: 102-32-9
Synonyms:
Catechol-4-acetic Acid;
Benzeneacetic acid, 3,4-dihydroxy-;
3,4-dihydroxy-1-benzeneacetic acid;
Chemical & Physical Properties:
Appearance: Beige to light brown crystalline powder
Assay :≥99.00%
Density: 1.478g/cm3
Boiling Point: 418.4℃ at 760mmHg
Melting Point: 126-130℃
Flash Point: 221℃
Refractive Index: 1.628
Water Solubility: Soluble in water and methanol
Stability: Stable under normal temperatures and pressures
Storage Condition: -20℃
Vapor Pressure: 0mmHg at 25℃
Safety Information:
RTECS: AH0590000
Safety Statements: S26-S36
HS Code: 2918290000
WGK Germany: 3
Risk Statements: R36/37/38
Hazard Code: Xi
Symbol: GHS07
Signal Word: Warning
Hazard Declaration: H315; H319; H335
Caution Statements: P261; P305 + P351 + P338
3,4-Dihydroxyphenylacetic acid (DOPAC) is a metabolite of the neurotransmitter dopamine.
Dopamine can be metabolized into one of three substances. One such substance is DOPAC. Another is 3-methoxytyramine (3-MT). Both of these substances are degraded to form homovanillic acid (HVA). Both degradations involve the enzymes monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT), albeit in reverse order: MAO catalyzes dopamine to DOPAC, and COMT catalyzes DOPAC to HVA; whereas COMT catalyzes dopamine to 3-MT and MAO catalyzes 3-MT to HVA. The third metabolic end-product of dopamine is norepinephrine (noradrenaline).
DOPAC can be oxidized by hydrogen peroxide, leading to the formation of toxic metabolites which destroy dopamine storage vesicles in the substantia nigra. This may contribute to the failure of levodopa treatment of Parkinson”s disease. A MAO-B inhibitor such as selegiline or rasagiline can prevent this from happening.
Biodegradation of dopamine; It can also be found in the bark of Eucalyptus globulus.
This product has been synthesized (52% yield) from 4-Hydroxyphenylacetic acid via aerobic biotransformation using whole cell cultures of Arthrobacter protophormiae.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:Bis(N-piperidinomonocarbamato)nickel(II) manufacturer | Benzotriazol-1-ylmethyl-phenoxathiin-2-yl-amine supplier | 5,11,17-trimethyl-5,6,11,12,17,18-hexahydro-cyclonona[1,2-b;4,5-b’;7,8-b’]triindole producer.
| Product Name | |
|---|---|
| 5-Formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid | View Details |
| Butyl methanesulfonate | View Details |
| CAS:18293-54-4 Evonik Degussa Dynasylan TMSTA | View Details |