

We are Dapoxetine Hydrochloride CAS:129938-20-1 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description:
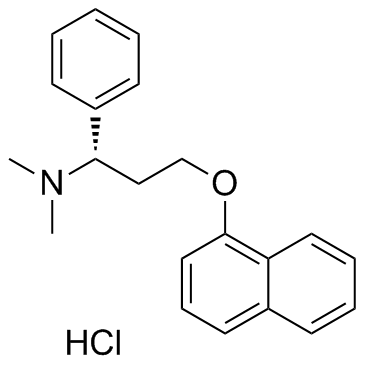
Product Name: Dapoxetine hydrochloride CAS NO: 129938-20-1
Synonyms:
(1S)-N,N-dimethyl-3-naphthalen-1-yloxy-1-phenylpropan-1-amine,hydrochloride;
(S)-N,N-Dimethyl-α-[2-(1-naphthyloxy)ethyl]benzylamine Hydrochloride;
(S)-N,N-Dimethyl-α-[2-(1-naphthyloxy)ethyl]benzylamine Hydrochloride;
Chemical & Physical Properties:
Appearance: White to off-white crystalline solid
Assay :≥99.00%
Boiling Point: 454.4℃ at 760 mmHg
Melting Point: 175-179℃
Flash Point: 132.6℃
Vapor Pressure: 1.27E-09mmHg at 25℃
Storage Condition: -20℃ Freezer
Safety Information:
HS Code: 2922199090
Hazard Declaration: H302; H319; H413
Signal Word: Warning
Caution Statements: P305 + P351 + P338
Symbol: GHS07
Hazard Codes: Xn,N
Dapoxetine, marketed as Priligy and Westoxetin, among and other brands, is the first compound developed specially for the treatment of premature ejaculation (PE) in men 18–64 years old. Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin”s action at the post synaptic cleft, and as a consequence promoting ejaculatory delay. As a member of selective serotonin reuptake inhibitor (SSRI) family, dapoxetine was initially created as an antidepressant. However, unlike other SSRIs, dapoxetine is absorbed and eliminated rapidly in the body. Its fast acting property makes it suitable for the treatment of PE but not as an antidepressant.
Originally created by Eli Lilly pharmaceutical company, dapoxetine was sold to Johnson & Johnson in 2003 and submitted as a New Drug Application to the Food and Drug Administration (FDA) for the treatment of PE in 2004. Dapoxetine has been sold in several European and Asian countries, and lately in Mexico. In the US, dapoxetine has been stuck in phase III development since 2003. However, it is expected to be marketed soon. In 2012, Menarini acquired the rights to commercialise Priligy in Europe, most of Asia, Africa, Latin America and the Middle East.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:(S)-2-(triphenylstannyl)propan-1-ol manufacturer | 18-phenyloctadecan-1-ol supplier | 3-anilino-5-benzylthio-4H-1,2,4-triazole producer.
| Product Name | |
|---|---|
| 2-Ethyl-3-methoxypyrazine | View Details |
| 2-Fluoro-N-methylaniline | View Details |
| disperse red 60 Cas:17418-58-5 | View Details |