


We are Cupric Nitrate,Basic CAS:12158-75-7 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product name:
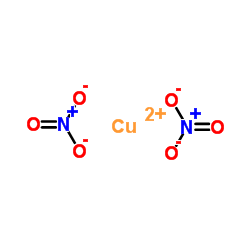
Cupric Nitrate,Basic
CAS No.: 12158-75-7
Cu(NO3)2·3Cu(OH)2
Molecular Weight: 480.22
Characters: Light blue powder, insoluble in water and alcohols, soluble in diluted acid and ammonia water.
Specifications:
| Cupric Nitrate,Basic | Special-purpose grade | Technical grade |
| Item | Spec. | Spec. |
| Assay(as Cu) % | 52.5-53.6 | 52.5-53.6 |
| Nitrates(NO3) % | 25.3-26.3 | 25.3-26.3 |
| Na% | ≤0.5 | ≤0.5 |
| Water insoluble% | ≤0.3 | ≤0.3 |
| pH-value(100g/L) | 5.5-7.5 | 5.5-7.5 |
| Granularity(UM) | D50≤1.5 | — |
Product Usage: It is used for new-generation auto airbag.
Packaging: In 25Kg/woven bag lined with double-layer plastic bag, closed tightly.
Storage Precautions: The toxic chemicals. Avoid inhalation of the product using the dust, avoid contact with eyes and skin. Should be sealed in dry place.
Related News: High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.(3-chlorophenyl)-(3,4-dimethoxyphenyl)methanone CAS:116412-84-1 High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.2-Methoxy-4-methylpyridine High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.2,3-dimethylbenzoic acid CAS:603-79-2 High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.
| Product Name | |
|---|---|
| 8-[(1R)-1-Hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]amino]ethyl]-6-(phenylmethoxy)-2H-1,4-benzoxazin-3(4H)-one Cas:869478-13-7 | View Details |
| 2-(4-(2-(4,4-dimethyl-4,5-dihydrooxazol-2-yl)propan-2-yl)phenyl)ethanol | View Details |
| 2-Chloro-4-fluoroiodobenzene Cas:101335-11-9 | View Details |