


We are Calcium beta-hydroxy-beta-methylbutyrate CAS:135236-72-5 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
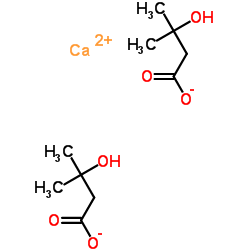
Product Name: Calcium beta-hydroxy-beta-methylbutyrate
Synonym: β-Hydroxy-β-methylbutyric acid calcium salt;Calcium β-hydroxy-β-methylbutyric acid;3-Hydroxyisovaleric acid calcium salt;HMB-Ca
Cas No.: 135236-72-5
Formula: C10H18CaO6
MW: 274.32
EINECS No.:681-140-5
Boiling point: 242.8ºC at 760 mmHg
Flash point: 114.9ºC
HS Code: 2918190090
UN No.: 1992
Hazard class: 3 categories
Packing level: Class II
Specification
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | White crystalline powder | White crystalline powder |
| Assay | ≥99.0% | 101.6% |
| HMB Assay | ≥84.0% | 85.88% |
| Ca2+ Assay | 13.5-15.5% | 14.77% |
| Loss on drying | ≤7.50% | 4.09% |
| Heavy metals ,(Pb) | ≤10ppm | <10ppm |
| As | ≤1ppm | <1ppm |
| Total Aerobic | 1000cfu/g max | <1000cfu/g |
| Mold and Yeast | 100cfu/g max | <100cfu/g |
| E.COLI form | Non-detective | Non-detective |
| Salmonella | Non-detective | Non-detective |
| Conclusion | Conforms to Factory Standard | |
Application
Applicationd as pharmaceutical intermediates, feed additives, etc.
Packaging
25 kg/barrel, can also be packaged according to customer requirements.
Storage
Store in a cool, ventilated warehouse.
Keep away from fire and heat.
The temperature should not exceed 37 °C.
Related News: This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved.3-Fluoro-2-methylbenzaldehyde CAS:147624-13-3 This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved.4-hidroxibenzotioamida CAS:25984-63-8 In August, the Company announced that the U.S. Patent and Trademark Office issued U.S. Patent No. 10,370,452 covering compositions and uses of effector T cells expressing a CAR, where such T cells are derived from a pluripotent stem cell, including an iPSC.5-Bromo-2-fluorobenzoic acid CAS:146328-85-0 Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations.Complete the research and development of APIs and intermediates, design new patented process routes, and explore new crystal forms.
| Product Name | |
|---|---|
| pentane-2,3-dione | View Details |
| (3R)-3-aminoazepan-2-one | View Details |
| METHYL THIOPROPIONATE Cas:5925-75-7 | View Details |