


We are BOC-L-Proline CAS:15761-39-4 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
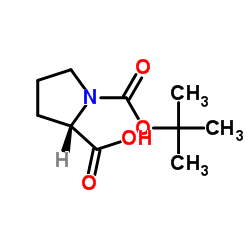
Chemical Name: BOC-L-Proline
CAS.NO:15761-39-4
Synonyms:Boc-L-Proline
N-(tert-Butyloxycarbonyl)-L-proline
N-(tert-Butoxycarbonyl)-L-proline
(S)-1-(tert-Butoxycarbonyl)pyrrolidine-2-carboxylic acid
N-Boc-L-proline
Molecular Formula: C10H17NO4
Molecular Weight:215.24600
Physical and Chemical Properties:
Density: 1.201 g / cm3
Boiling point: 337.2ºC at 760 mmHg
Melting point: 133-136ºC
Flash point: 157.7ºC
Refractive index: 1.503
Specification:
Appearance:White to off-white crystalline powder
Purity:≥99.0%
Specification:A white to off-white crystalline powder
Identification by HPLC:The retention time of major peak in the chromatogram of the sample solution corresponds to that in the chromatogram of the standard solution, as obtained in the purity.
Melting range:Between 132 and 137ºC
Water by K.F.:Not more than 0.5% w/w
Chromatographic purity (%area normalization, by HPLC)
1)Purity
2)Total impurities
:Not less than 99.0% Not more than 1.0%
Packing:
25kg / drum
Storage:Closed, cool and dry place below 0ºC.
Application: It can be used to synthesize Daclatasvir which inhibits the HCV protein NS5A, and thus can be used as a drug candidate for the treatment of hepatitis C (HCV).Intermediates of Daclatasvir CAS:1009119-64-5
Related News: In 2016, the export volume of China’s specialty drug substances reached US $ 3.53 billion, accounting for 13.8% of the total drug substances.4-bromo-2-cloropiridina CAS:73583-37-6 Any drug or medication is composed of two components.212322-56-0 Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.4-Bromo-4'-iodo-1,1'-biphenyl Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.API (Active Pharmaceutical Ingredient) means the active ingredient which is contained in medicine.
| Product Name | |
|---|---|
| 1-Iodo-2,2,3,3-tetrafluoropropane | View Details |
| (2S)-5-methoxy-1,2,3,4-tetrahydronaphthalen-2-amine | View Details |
| ALKYL (C12-C14) GLYCIDYL ETHER Cas:68609-97-2 | View Details |