


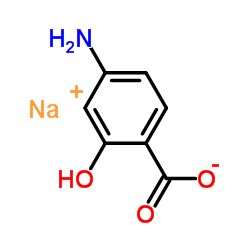
We are Aminosalicylate Sodium CAS:133-10-8 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: What is the difference? Raw material refers to chemical compounds that are used as a base to make an API.50606-58-1 The oral form of rigosertib was developed to provide a potentially more convenient dosage form for use where the duration of treatment may extend to multiple years.351-50-8 With the speeding up of review and approval, the number of approved innovative drugs in China is about to usher in a year of blowout.3-Bromo-4-fluorobenzotrifluoride In recent years, China’s bulk drug companies have gradually completed the upgrade of the product structure of bulk raw materials to specialty raw materials and intermediates. The industry’s leading companies have further developed the research and development layout of high-barrier generic pharmaceutical raw materials with multiple patents that have not yet expired.With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand.
| Product Name | |
|---|---|
| 4-Amino-3-iodobenzotrifluoride | View Details |
| CAS:115-19-5 3-Methyl butynol | View Details |
| Trifluoroacetic anhydride/TFAA Cas:407-25-0 | View Details |