


We are Esomeprazole Magnesium Trihydrate CAS:217087-09-7 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Product Description:
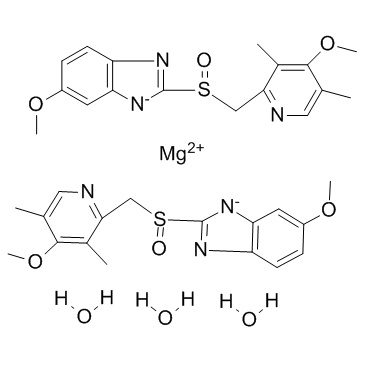
Product Name: Esomeprazole magnesium trihydrate CAS NO: 217087-09-7
Synonyms:
Esomeprazole magnesium;
(S)-Omeprazole magnesium trihydrate;
5-methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]benzimidazol-1-ide;
Chemical & Physical Properties:
Appearance: Off-white powder
Assay :≥99.0%
Boiling Point: 600℃ at 760 mmHg
Melting Point: 184-189℃ (dec.)
Flash Point: 316.7℃
Vapor Pressure: 2.35E-14mmHg at 25℃
StorageTemp: -20℃ Freezer
Solubility: Slightly soluble in water, soluble in methanol, practically insoluble in heptane.
Safety Information:
Hazard Code: Xn
Risk Statements: R22
WGK Germany: 3
HS Code: 2933399090
S-Form of Omeprazole. Gastric proton-pump inhibitor.
esomeprazole magnesium trihydrate for the healing of peptic ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy.
Zollinger-Ellison syndrome
Peptic ulcer, site unspecified
Duodenal ulcer
Gastric ulcer
Gastro-oesophageal reflux disease
Esomeprazole belongs to the family of medications known as proton pump inhibitors (PPIs). Esomeprazole is registered by the Therapeutic Goods Administration (TGA) for the following indications:
Gastro-Oesophageal Reflux Disease (GORD):
treatment of erosive reflux oesophagitis;
long-term management of patients with healed oesophagitis to prevent relapse;
symptomatic treatment of gastro-oesophageal reflux disease (GORD).
In combination with appropriate antibiotics for:
healing of duodenal ulcer associated with Helicobacter pylori;
eradication of Helicobacter pylori in patients with active or healed peptic ulcer.
Short-term treatment of upper gastrointestinal symptoms associated with non-steroidal anti-inflammatory drug (NSAID) (non-selective and COX-2 selective) therapy.
Healing of gastric ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy.
Prevention of gastric and duodenal ulcers associated with non-steroidal anti-inflammatory drug NSAID (non-selective and COX-2 selective) therapy in patients at risk.
If you are interested in our products or have any questions, please feel free to contact us!
Products under patent are offered for R & D purpose only. However, the final responsibility lies exclusively with the buyer.
Related Products:N-(3-anilino-5-methylsulfanyl-[1,2,4]triazol-4-yl)-N’,N’-diphenyl-guanidine manufacturer | 2-(5-(hydroxy-24-trioxidaneyl)-13,34,43,54-pentaoxol-3-yl)-6-tetraoxidaneyl-24,33,44,53,64,73,83,94-pentaoxolo[2,1]hexaoxine supplier | (2-hydroxy-2-oxido-1,3,2-dioxaphospholan-4-yl)methyl palmitate producer.
| Product Name | |
|---|---|
| OCTENIDINE HYDROCHLORIDE Cas:70775-75-6 | View Details |
| 1,2-BIS(TETRABROMOPHTHALIMIDO) ETHANE Cas:32588-76-4 | View Details |
| palmitoylethanolamide Cas:544-31-0 | View Details |