


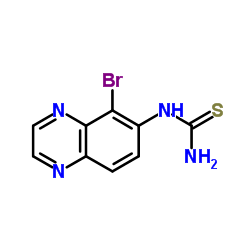
We are 5-Bromoquinazolin-6-ylthiourea CAS:842138-74-3 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: In a stringent xenograft model of disseminated lymphoblastic leukemia, FT819 demonstrated enhanced tumor clearance and control of leukemia as compared to primary CAR19 T cells.5-bromo-3-nitro-2-piridinol CAS:15862-34-7 From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.1214369-42-2 From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.1-cloro-8-fluorooctano CAS:593-14-6 From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.Due to the time-consuming process of EIA approval and EIA acceptance for newly-built projects, and environmental protection supervision policies are becoming increasingly tight, the company strives to improve capacity utilization through reasonable equipment layout when designing and constructing capacity.
| Product Name | |
|---|---|
| 4-[(5,6-Diphenyl-2-Pyrazinyl)(1-Methylethyl)Amino]-1-Butanol Cas:475086-75-0 | View Details |
| 4-(4-Bromo-3-formylphenoxy)benzonitrile | View Details |
| Tetrahydropapaverine hydrochloride Cas:6429-04-5 | View Details |