


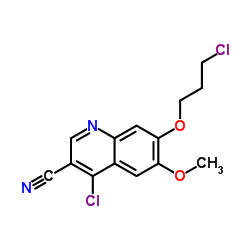
We are 4-Chloro-7-(3-chloropropoxy)-6-methoxyquinoline-3-carbonitrile CAS:214470-68-5 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Any drug or medication is composed of two components.4,6-Dichloro-2-methylpyrimidine Trial results demonstrated that Oligomannate statistically improved cognitive function in mild-to-moderate AD patients as early as week 4 and the benefit was sustained at each follow-up assessment visit,” Shanghai Green Valley Pharmaceuticals, which developed the drug along with two academic institutions in China, said in a statement.(S)-(-)-1,2,3,4-Tetrahydroisoquinoline-3-Carboxylic Acid CAS:74163-81-8 Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.2-Isopropylphenol The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.
| Product Name | |
|---|---|
| Bromo(tri-tert-butylphosphine)palladium(I) Dimer Cas:185812-86-6 | View Details |
| 2-Fluoro-3-chlorotoluene Cas:85089-31-2 | View Details |
| HC Blue 7 | View Details |