


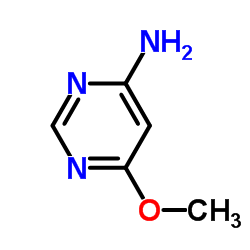
We are 4-Amino-6-methoxypyrimidine CAS:696-45-7 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: The move could force international drugmakers to further cut prices and enable copycat medicines to replace imported off-patent brands at faster pace.Trimethoxysilane The move could force international drugmakers to further cut prices and enable copycat medicines to replace imported off-patent brands at faster pace.Bis (diisopropilamino) (2-cianoetoxi) fosfina CAS:102691-36-1 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.2-((4-Chlorophenyl)(piperidin-4-yloxy)methyl)pyridine CAS:122368-54-1 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.At the same time, due to the relatively late development of the bulk drug business in China, most of the established bulk drug companies are currently in the field of commonly used generic drug bulk drugs. Stand up.
| Product Name | |
|---|---|
| Iron(III) Nitrate nonahydrate Cas:7782-61-8 | View Details |
| IRAK Inhibitor 6 Cas:1042672-97-8 | View Details |
| 3-bromo-2-fluorobenzaldehyde | View Details |