


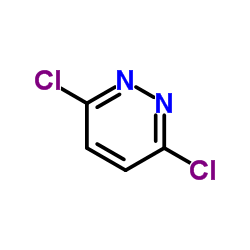
We are 3,6-Dichloropyridazine CAS:141-30-0 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Pharmaceutical intermediates: chemical raw materials or chemical products used in the process of pharmaceutical synthesis, are intermediate products in the process of producing APIs, and can be further processed into APIs.triphenylene Pharmaceutical intermediates: chemical raw materials or chemical products used in the process of pharmaceutical synthesis, are intermediate products in the process of producing APIs, and can be further processed into APIs.4-cloro-3-fluorobenzonitrilo CAS:110888-15-8 The upstream industry for the R & D, production and sales of pharmaceutical intermediates is the basic chemical raw material industry, and the downstream industry is the chemical bulk drug and chemical pharmaceutical preparation industry.1-fluoro-9-yododecano CAS:/ “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.“Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.
| Product Name | |
|---|---|
| 2,4-dihydroxybenzophenone Cas:131-56-6 | View Details |
| 3-[2-(Perfluorohexyl)ethoxy]-1,2-epoxypropane Cas:122193-68-4 | View Details |
| SUCCINONITRILE Cas:110-61-2 | View Details |