


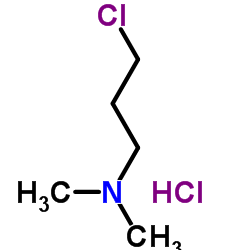
We are 3-Dimethylaminopropylchloride hydrochloride CAS:5407-04-5 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.2882-20-4 Due to the time-consuming process of EIA approval and EIA acceptance for newly-built projects, and environmental protection supervision policies are becoming increasingly tight, the company strives to improve capacity utilization through reasonable equipment layout when designing and constructing capacity.34036-80-1 In the early stage of market development, API companies usually need to strive to become a supply company for pharmaceutical companies after quickly completing product preparations. In the subsequent scale-up of production and market application, the relationship between pharmaceutical companies and API companies will become closer. The choice of R & D products of pharmaceutical companies will also be affected by pharmaceutical companies in turn.958-09-8 The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.ICIG will also supply materials needed for the manufacture of other treatments in earlier stages of development, including neo-GAA, currently in preclinical development as a potential next-generation Pompe disease therapy.
| Product Name | |
|---|---|
| Ethyl 4,4,4-Trifluorobutyrate Cas:371-26-6 | View Details |
| 1-BOC-piperidine-4-carboxamide Cas:91419-48-6 | View Details |
| Cinnamaldehyde Cas:104-55-2 | View Details |