


We are (2R)-6-fluoro-3,4-dihydro-2H-chromene-2-carboxylic acid CAS:129101-37-7 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
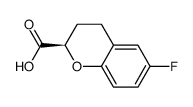
Chemical Name: (2R)-6-fluoro-3,4-dihydro-2H-chromene-2-carboxylic acid
CAS.NO:129101-37-7
Synonyms:
(2R)-6-fluoro-3,4-dihydro-2H-chromene-2-carboxylic acid
Molecular Formula: C10H9FO3
Molecular Weight: 196.17500
Physical and Chemical Properties:
Density:1.364g/cm3
Boiling point: 358ºC at 760 mmHg
Melting point: /
Flash point: 170.3ºC
Refractive index: 1.554
Specification:
Appearance: White solid
Purity:≥99%
Packing:
25kg cardboard drum or according to customer specified requirements
Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application:
Intermediates of Nebivolol hydrochloride CAS: 152520-56-4
Related News: Any drug or medication is composed of two components.4-(2,3-Dichlorophenyl)-5-(methoxycarbonyl)-2,6-dimethyl-1,4-dihyd ro-3-pyridinecarboxylic acid Any drug or medication is composed of two components.quinoxalin-2-ol CAS:1196-57-2 Because API companies usually adopt a different synthetic route from the original research company, compared with the drug itself, the impurities contained in the generic drug API have not been verified by the original drug for many years of use and require more careful testing and control.Dichloromethylphenylsilane Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.
| Product Name | |
|---|---|
| pentafluorophenol Cas:771-61-9 | View Details |
| Optical Brightener FP127 | View Details |
| 1-Methoxypyrene Cas:34246-96-3 | View Details |