


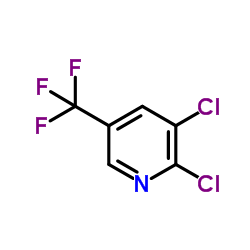
We are 2,3-Dichloro-5-(trifluoromethyl)pyridine CAS:69045-84-7 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: In 2016, the export volume of China’s specialty drug substances reached US $ 3.53 billion, accounting for 13.8% of the total drug substances.61-19-8 Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.Bromuro de 4-fenoxibutilo CAS:1200-03-9 Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance.3-Amino-2,2-dimethylpropanamide CAS:324763-51-1 For example, an active ingredient to relieve pain is included in a painkiller.INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.
| Product Name | |
|---|---|
| HYPOCRELLIN B Cas:123940-54-5 | View Details |
| 5-Bromo-7-azaindole | View Details |
| [(1S,3S,4S)-4-Amino-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-carbamicacid 1,1-dimethylethyl Ester Cas:144163-85-9 | View Details |