


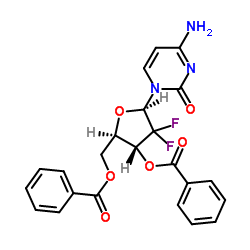
We are 2′,2′-Difluoro-2′-deoxycytidine-3′,5′-dibenzoate CAS:134790-39-9 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to the development of first-in-class cellular immunotherapies for cancer and immune disorders.77-55-4 Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to the development of first-in-class cellular immunotherapies for cancer and immune disorders.4-bromobutan-1-ol From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend.2,3-Difluoro-6-methylpyridine CAS:1227579-04-5 This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?
| Product Name | |
|---|---|
| 4-Methoxyphenethylamine Cas:55-81-2 | View Details |
| Glutathione | View Details |
| 2-acetamido-2-(4-octanoylphenethyl)propane-1,3-diyl Diacetate Cas:249289-07-4 | View Details |