


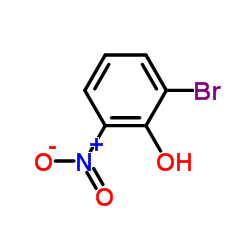
We are 2-Bromo-6-nitrophenol CAS:13073-25-1 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).4-methylsalicylic acid The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.944801-28-9 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.Ácido (2-metoxinaftalen-1-il) borónico CAS:104116-17-8 The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.
| Product Name | |
|---|---|
| 2-HYDROXY-3-BUTANONE Cas:513-86-0 | View Details |
| (2S)-2-[[(2S)-2-aminopropanoyl]amino]-3-phenylpropanoic acid | View Details |
| titanium tetraisopropanolate Cas:546-68-9 | View Details |