

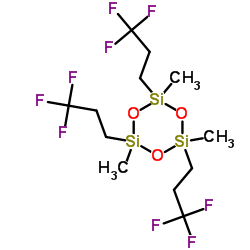
We are 1,3,5-Tris(3,3,3-trifluoropropyl)methylcyclotrisiloxane CAS:2374-14-3 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.3-BAP2NA-B CAS:944801-33-6 The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.rac-1-(4-Fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile CAS:64169-67-1 The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.42518-98-9 ICIG will also supply materials needed for the manufacture of other treatments in earlier stages of development, including neo-GAA, currently in preclinical development as a potential next-generation Pompe disease therapy.ICIG will also supply materials needed for the manufacture of other treatments in earlier stages of development, including neo-GAA, currently in preclinical development as a potential next-generation Pompe disease therapy.
| Product Name | |
|---|---|
| 1,2,3,4-Tetrahydro-benzo[b]azepin-5-one | View Details |
| 5-pyrrol-1-yl-1,3-dihydrobenzimidazole-2-thione Cas:172152-53-3 | View Details |
| 4-cyclopropylnaphthalen-1-amine Hydrochloride Cas:1533519-92-4 | View Details |