


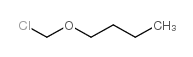
We are 1-(chloromethoxy)butane CAS:2351-69-1 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: APIs are generally manufactured through a variety of processes that include.[2 – [(2-amino-6-oxo-3H-purin-9-il) metoxi] -3-hidroxipropil] acetato CAS:88110-89-8 Due to the time-consuming process of EIA approval and EIA acceptance for newly-built projects, and environmental protection supervision policies are becoming increasingly tight, the company strives to improve capacity utilization through reasonable equipment layout when designing and constructing capacity.111-31-9 In an August overhaul to its drug administration law, Beijing said conditional approval could be granted to some still-under-research medicines of “predictable” clinical value for life-threatening diseases for which effective treatment is not immediately available.arsorosomethane In an August overhaul to its drug administration law, Beijing said conditional approval could be granted to some still-under-research medicines of “predictable” clinical value for life-threatening diseases for which effective treatment is not immediately available.The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.
| Product Name | |
|---|---|
| 1,3-Bis(2,4-Diaminophenoxy)Propane 4HCl | View Details |
| 2-AMINOTOLUENE-5-SULFONIC ACID Cas:98-33-9 | View Details |
| 4-Chloro-4′-hydroxybenzophenone Cas:42019-78-3 | View Details |