


We are 1-(4-Hydrazinylphenyl)-N-methylmethanesulfonamide CAS:139272-29-0 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
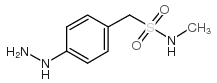
Chemical Name: 1-(4-Hydrazinylphenyl)-N-methylmethanesulfonamide
CAS.NO: 139272-29-0
Synonyms:
4-Hydrazino-N-methylbenzenemethanesulfonamide
Molecular Formula: C8H13N3O2S
Molecular Weight: 215.27300
Physical and Chemical Properties:
Density: 1.356 g/cm3
Boiling point: 427.4ºC at 760 mmHg
Melting point: /
Flash point: 212.3ºC
Refractive index: 1.619
Specification:
Appearance: powder
Purity:≥98%
Packing:
25kg cardboard drum or according to customer specified requirements
Storage: Refrigerator.Stored in a cool and dry well-closed container. Keep away from moisture and strong light/heat.
Application:
Intermediates of Sumatriptan succinate CAS:103628-48-4
Intermediates of Sumatriptan CAS:103628-46-2
Related News: The first is the API – which is the central ingredient.o-Toluoyl chloride CAS:933-88-0 Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.124750-51-2 The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.168267-41-2 The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved.
| Product Name | |
|---|---|
| dipropoxy-p-toluidine Cas:38668-48-3 | View Details |
| benzyl (2S)-2-amino-3-methylbutanoate,hydrochloride | View Details |
| 2-Fluoro-5-(trifluoromethyl)pyridine | View Details |