


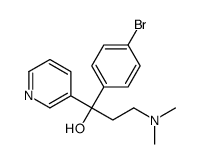
We are 1-(4-bromophenyl)-3-(dimethylamino)-1-pyridin-3-ylpropan-1-ol CAS:41910-98-9 manufacturer and supplier in China, Pls send inquiry of to info@nbinno.com of visit www.nbinno.com our official website should you have any interests
Related News: At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world.4-amino-1-piperidinacarboxilato de etilo CAS:58859-46-4 At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world.(4Z)-4-[[2-methoxy-5-methyl-4-(methylsulfamoyl)phenyl]hydrazinylidene]-3-oxo-N-(2-oxo-1,3-dihydrobenzimidazol-5-yl)naphthalene-2-carboxamide CAS.NO:51920-12-8 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.80-62-6 This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.
| Product Name | |
|---|---|
| 1-Methyl-1H-imidazole-2-carboxylic acid | View Details |
| N-OCTYL ACRYLATE | View Details |
| Potassium Ferricyanide Cas:13746-66-2 | View Details |